Melanoma, the most fatal type of skin cancer, is characterized by oncogenic mutations driving the MAPK signaling pathway, crucial for cancer cell proliferation and survival. While targeted therapies inhibiting BRAF or MEK kinases show efficacy, resistance frequently emerges. The Ortiz Lab focuses on uncovering molecular targets to enhance MAPK pathway inhibition or thwart alternate pathways circumventing MAPK therapy effects, prioritizing biomedical advancement in melanoma treatment.
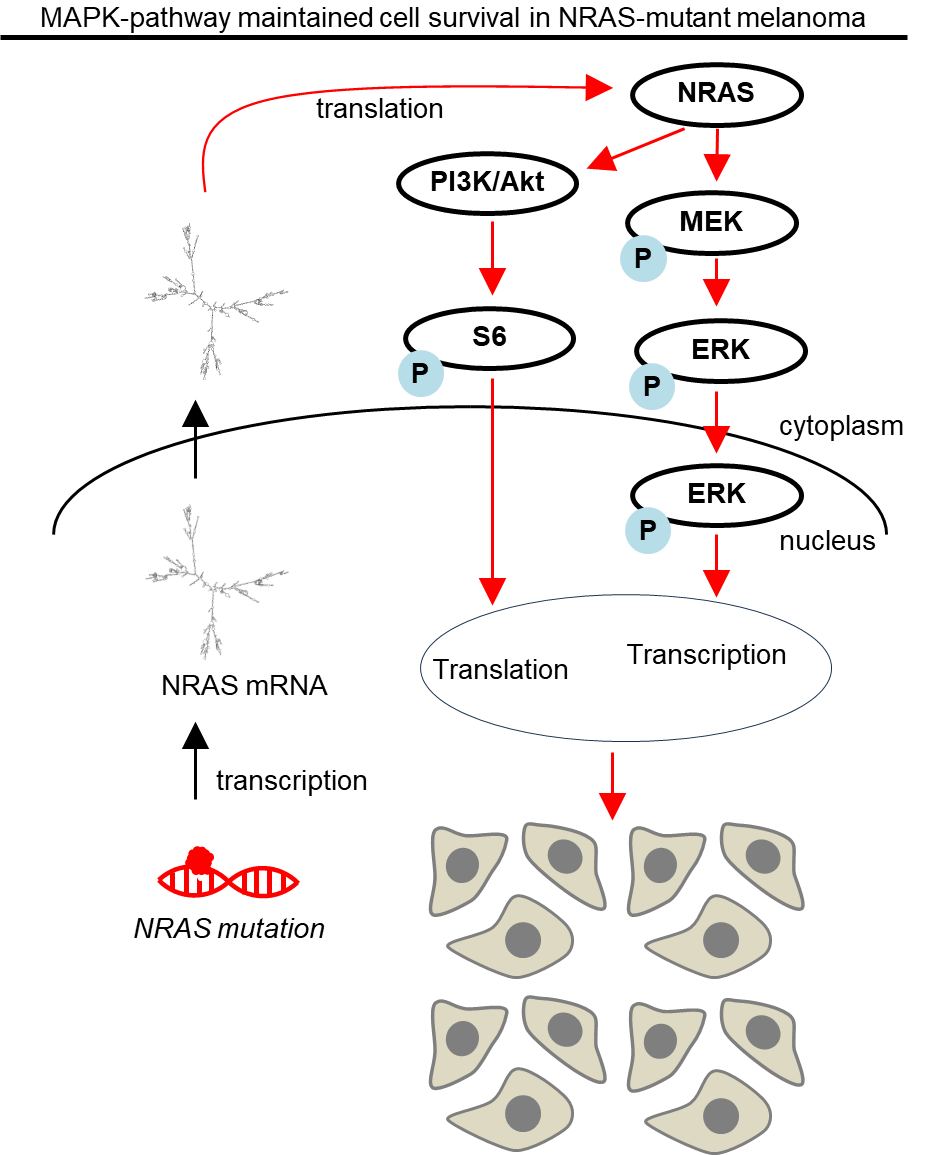
A large fraction of our research focuses on the subtype of NRAS-mutant melanoma:
NRAS-driven melanomas are characterized by unique clinical features, such as thicker tumors, higher rates of occurrence on extremities, rapid onset of treatment resistance, higher mitotic index, and lower ulceration rates. Ultimately these factors lead to poor prognosis.
Our laboratory has successfully identified drug combinations that effectively inhibit the growth of NRAS-mutant melanoma cells and tumors, as showcased by Posch et al (https://pubmed.ncbi.nlm.nih.gov/23431193) and Vujic et al (https://pubmed.ncbi.nlm.nih.gov/25504439). Additionally, we have addressed a specific form of melanoma occurring in the eye, known as uveal melanoma. This was achieved by connecting pharmaceutical compounds to nanoparticles to improve delivery and stability (https://pubmed.ncbi.nlm.nih.gov/25653058) and (https://pubmed.ncbi.nlm.nih.gov/24496380).
Furthermore, we have uncovered and characterized a crucial long non-coding RNA (lncRNA) transcript, named MIRAT, which plays a pivotal role as a regulator for drug resistance in melanoma (https://pubmed.ncbi.nlm.nih.gov/30026510).
Expanding on these groundbreaking findings that underscore the significance of lncRNAs in melanoma, our research delved into the specific role of the oncogenic lncRNA MALAT1 in NRAS-mutant melanoma. This exploration revealed critical regulatory functions that MALAT1 assumes in the context of NRAS-mutant melanoma, emphasizing the substantial impact of lncRNAs in this setting. (https://pubmed.ncbi.nlm.nih.gov/37235843)
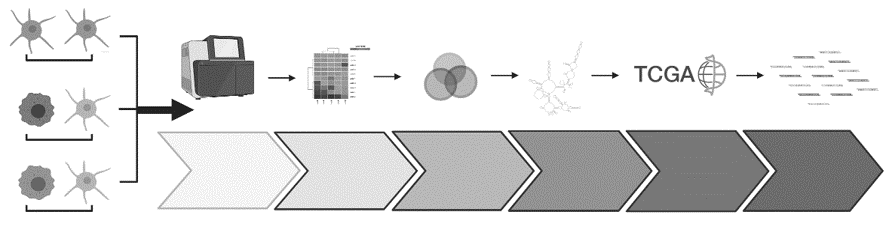
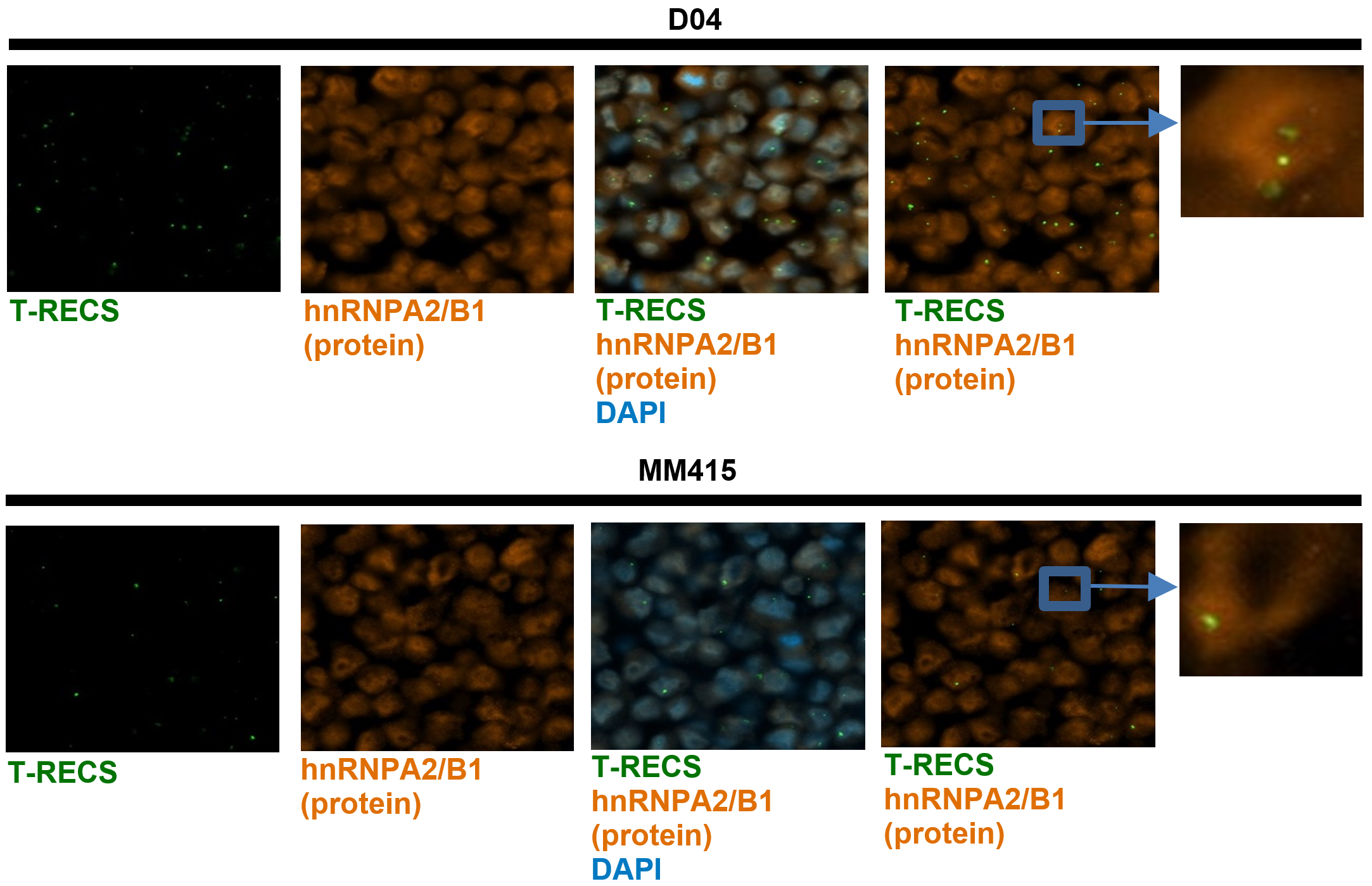
Continuing our investigation, we recognized the pivotal role of lncRNAs in melanoma and developed a comprehensive platform. This platform incorporated healthy skin cells, cells mimicking early cancer states, melanoma cells, and data from hundreds of melanoma patients. The outcome was a robust pipeline designed to identify lncRNAs that are specifically associated with melanoma (https://pubmed.ncbi.nlm.nih.gov/38383439).
Through this pipeline, we identified several potential targets, with the oncogenic lncRNA T-RECS standing out prominently. Based on the results of our platform we founded Spartia Therapeutics, a company dedicated to developing RNA-based cancer therapies that can improve outcomes for patients. Through our research and development efforts, we hope to make a significant impact on the lives of those affected by cancer (https://pubmed.ncbi.nlm.nih.gov/38383439).